Page 8 - April-Month
P. 8
TECHNICAL PAPER
2200 2400
2000
2200
Chloride concentration (ppm) 1800 Chloride concentration (ppm) 2000
1800
1600
1600
1400
1200
1000 1400
1200
10 20 30 40 50 10 20 30 40 50
Depth (mm) Depth (mm)
th
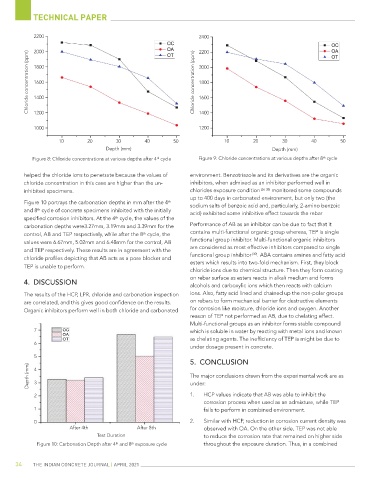
Figure 8: Chloride concentrations at various depths after 4 cycle Figure 9: Chloride concentrations at various depths after 8 cycle
th
helped the chloride ions to penetrate because the values of environment. Benzotriazole and its derivatives are the organic
chloride concentration in this case are higher than the un- inhibitors, when admixed as an inhibitor performed well in
inhibited specimens. chlorides exposure condition [36-38] monitored some compounds
up to 400 days in carbonated environment, but only two (the
Figure 10 portrays the carbonation depths in mm after the 4 sodium salts of benzoic acid and, particularly, 2-amino benzoic
th
and 8 cycle of concrete specimens inhibited with the initially acid) exhibited some inhibitive effect towards the rebar
th
specified corrosion inhibitors. At the 4 cycle, the values of the
th
carbonation depths were3.27mm, 3.19mm and 3.39mm for the Performance of AB as an inhibitor can be due to fact that it
control, AB and TEP respectively, while after the 8 cycle, the contains multi-functional organic group whereas, TEP is single
th
values were 6.67mm, 5.02mm and 6.48mm for the control, AB functional group inhibitor. Multi-functional organic inhibitors
and TEP respectively. These results are in agreement with the are considered as most effective inhibitors compared to single
[39]
chloride profiles depicting that AB acts as a pore blocker and functional group inhibitor . ABA contains amines and fatty acid
TEP is unable to perform. esters which results into two-fold mechanism. First, they block
chloride ions due to chemical structure. Then they form coating
on rebar surface as esters reacts in alkali medium and forms
4. DISCUSSION alcohols and carboxylic ions which then reacts with calcium
The results of the HCP, LPR, chloride and carbonation inspection ions. Also, fatty acid lined and chained up the non-polar groups
are correlated, and this gives good confidence on the results. on rebars to form mechanical barrier for destructive elements
Organic inhibitors perform well in both chloride and carbonated for corrosion like moisture, chloride ions and oxygen. Another
reason of TEP not performed as AB, due to chelating effect.
Multi-functional groups as an inhibitor forms stable compound
7 which is soluble in water by reacting with metal ions and known
as chelating agents. The inefficiency of TEP is might be due to
6
under dosage present in concrete.
5
5. CONCLUSION
Depth (mm) 4 3 The major conclusions drawn from the experimental work are as
under:
2 1. HCP values indicate that AB was able to inhibit the
corrosion process when used as an admixture, while TEP
1 fails to perform in combined environment.
0 2. Similar with HCP, reduction in corrosion current density was
After 4th After 8th observed with OA. On the other side, TEP was not able
Test Duration to reduce the corrosion rate that remained on higher side
Figure 10: Carbonation Depth after 4 and 8 exposure cycle throughout the exposure duration. Thus, in a combined
th
th
34 THE INDIAN CONCRETE JOURNAL | APRIL 2021